The Plastics Plant is one of the country's leading manufacturers of modern industrial explosives. In particular, the company extracts explosives from decommissioned ammunition, and as a result of such disposal, they have learned to obtain a unique product – nanodiamond charge. Demand for it is growing all over the world, because nanodiamonds are widely used in various fields: from quantum electronics to medicine.
What are nanodiamonds, how they were discovered, where they are used today – read in our material.
Very "nano": for biomedicine and quantum electronics
By its chemical composition and structure, nanodiamonds are the same diamond. All the same carbon atoms packed in a "cubic" crystal lattice. As can be seen by the prefix "nano", such diamonds are very small in size. Recall that a nanometer is one billionth of a meter. Now you can imagine a particle with a size of only 4-6 nm – about the diameter of nanodiamonds. It is impossible to use them in jewelry, not only because of the size - nanodiamond is a nondescript powder of gray color. Why, instead of large and beautiful stones, grow synthetic diamonds that you can't even see?
To answer this question, we need to return to the structure of the diamond. As mentioned above, the diamond structure cell has the shape of a cube. To put it scientifically, the diamond crystallizes in the "syngony". This is the densest packing of atoms, hence the incredible strength for which diamonds are famous. So, in a diamond, each atom is surrounded by four atoms, each of which is surrounded by four more, and so on. According to this logic, it is clear that the extreme atoms on the surface will remain without an environment – in scientific terms, these are "uncompensated valences". Such "lonely" carbon atoms can bind with other atoms – hydrogen, oxygen. As a result, certain groupings of atoms – functional groups - are collected on the surface.
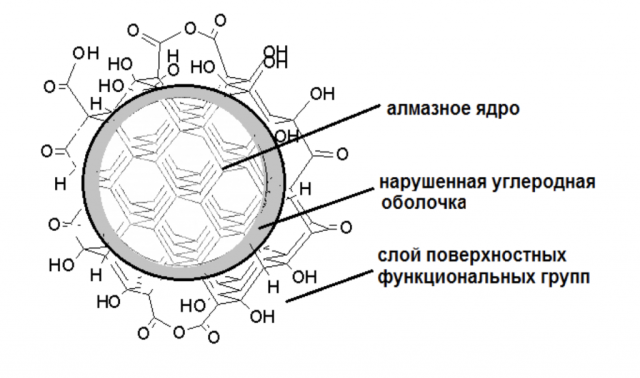
Source: Faculty of Chemistry, Moscow State University
Thus, each nanodiamond can be modified – various molecules can be "sewn" to its surface. This ability is currently used in various medical research, especially when it comes to the "targeted delivery" of drugs in the body. For example, nanodiamonds can be an excellent "transport" for antibiotics and deliver them exactly to the address. The size of such a particle with a diameter of only five nanometers allows it to safely pass through the cell membrane, so it is possible to deliver drugs even to individual cell organelles.
The second major area of application is quantum electronics and optics. To create quantum computers, a single-photon emitter is very important, which emits strictly one photon, that is, one quantum of light. Most modern single–photon emitters are various dyes that work only at very low temperatures, which is not very convenient. It turned out that nanodiamonds can become very good single-photon emitters even at room temperature.
Summarizing, we can say that nanodiamonds will definitely find a place in the XXI century – they can be used today in various fields, starting with biomedicine and ending with quantum electronics. However, the history of these small diamonds began in the last century and for many years nanodiamonds "worked" in industry – they were used for the oxidation of aluminum and its alloys, in the production of lubricating oils, microabrasive and polishing compounds, polymer compositions, rubbers, etc.
Explosive technology: from the history of the discovery of nanodiamonds
To date, several methods of synthesis of nanodiamonds are known. For example, the simplest and most obvious is to get from natural diamonds. However, the detonation method is most widely used, so sometimes nanodiamonds are called detonation diamonds. The principle is simple – if TNT or rdx is blown up in a special chamber, then a large amount of carbon is formed in the gas during the explosion and diamond particles can be found in the soot. Of course, it's not that simple and one explosion is not enough.
It is believed that the discoverers of this method were Soviet scientists. Moreover, this story is unique, since the synthesis of nanodiamonds was discovered three times in the USSR. In the 1960s and 1980s, a kind of "diamond club" of research institutes was formed in the country. One of the main participants was the All-Union Scientific Research Institute of Technical Physics (VNIITF), where nuclear munitions were developed. It was here in July 1963 that the detonation synthesis of nanodiamonds was first performed.

Nanodiamonds. Photo: D. Mukherjee / wikimedia.org
Back in the early 1960s, scientists came to the conclusion that if you raise the temperature and pressure, you can get a diamond from graphite, or from soot. Recall that graphite, which is familiar to everyone from pencil leads, is the same carbon, only in a slightly different form. Of course, heating the pencil lead alone does not turn it into a precious stone – the substance is important and strongly compressed.
VNIITF specialists were primarily engaged in the development of weapons. In particular, experiments were conducted with explosions of TNT and rdx in a closed volume, that is, in a metal ball or cylinder. It turned out that if the exploding object is cooled very quickly, and then the resulting soot is treated with nitric acid, which gradually dissolves graphite-like carbon, then diamond grains of several nanometers in size will remain.
Detonation synthesis of nanodiamonds turned out to be a very fast process compared to the long growing of crystals under pressure. But in the 1960s, there was no use for such small diamonds, the works were gradually closed. We returned to this topic only in the early 1980s. Then the synthesis of nanodiamonds was established in several scientific centers of the country at once, and ten years later pilot production began. The two enterprises produced approximately 50 kilograms of purified nanodiamonds per month.
Diamonds on "Plastic"
The Plastics plant from the city of Kopeysk, Chelyabinsk region specializes in the production of ammunition for naval guns, tank and artillery guns and some types of aircraft shells. At the same time, for many years, the company has been developing a direction for the disposal of ammunition – their number reached 100 thousand units per year. The projectile is disassembled, explosives and gunpowder are sent to the manufacture of industrial explosives, there is also scrap steel, non-ferrous metals such as copper and lead and their alloys.

The company's specialists have mastered the use of explosives from old ammunition and for the detonation synthesis of nanodiamonds. The plant has three blast chambers made of high-strength steel. The nanodiamond charge is obtained here by blasting in ice shells – the charge is immersed in an ice shell, and then placed in the cavity of the blast chamber. After the explosion, a charge is obtained – a wet mass, seemingly ordinary dirt, which is then separated and drained. The yield of the final product – nanodiamond – from such a charge is 8-10% of the mass of the exploded charge. Thus, precious nanodiamonds are born out of practically nothing – recyclable materials to be destroyed.

