Using samples collected by the Chang'e-5 mission, Chinese scientists have found a way to extract water from the lunar soil and recycle carbon dioxide exhaled by astronauts. This is done by using a small device powered by solar energy. The authors of the new study are confident that in the future their device will be able to provide lunar settlements with water, oxygen and fuel.
Humanity has long considered the Moon as a potential site for a habitable base. But shipping resources from Earth makes this project extremely expensive and difficult. Therefore, scientists around the world are looking for ways to provide future astronauts with air, water and fuel on site — using what is already on the satellite.
Lunar regolith, that is, the surface layer of dust and rocks covering the entire moon, contains a certain amount of water "trapped" inside minerals. However, the methods of extracting this water are characterized by high energy consumption and technological difficulties. Such approaches are difficult to apply in extreme lunar conditions.
Now, a team of chemists and engineers led by Lu Wang from the Chinese University of Hong Kong has found a solution that can greatly simplify the task.
Researchers have built a compact solar-powered installation using lunar soil and carbon dioxide exhaled by humans as raw materials. In the experiments, the scientists used real lunar samples delivered to Earth by the Chinese Chang 'e-5 mission in 2020, as well as their terrestrial counterparts (rocks and materials that resemble lunar samples in many ways in composition and texture).
The principle of operation of the device is quite simple. Solar heat heats the regolith to a temperature at which the water contained in it (both adsorption and bound in minerals) passes into the gas phase and becomes available for collection. Then a chemical reaction begins: water and carbon dioxide interact. The soil acts as a catalyst in this process.
The result is three important products: oxygen, hydrogen, and carbon monoxide. All of them can be used as fuel or as life support products. Oxygen is needed for respiration, hydrogen and carbon monoxide can be used as components of rocket fuel.
The main role in the chemical reaction, according to Wang, is played by a mineral called ilmenite (FeTiO3) — titanium ironstone. It is contained in the lunar regolith in noticeable quantities and is able to accelerate the necessary processes under the influence of sunlight. That is, the catalytic properties of ilmenite make the chemical reaction effective under sunlight without additional reagents or sophisticated equipment.
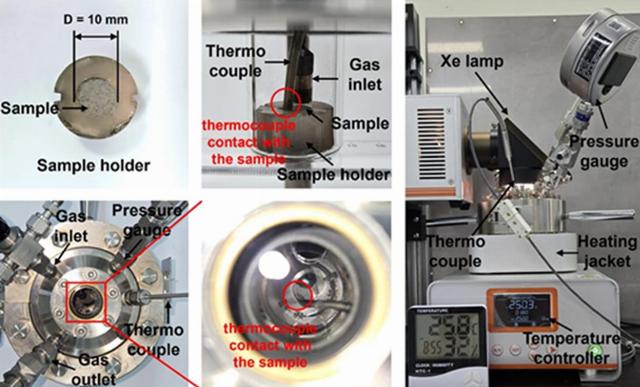
The Chinese installation. This is a sample that was tested in the laboratory.
Image source: Lu Wang
However, Wang admitted that the current device is just a compact sample demonstrating the idea. A full-size system capable of supporting a lunar colony is still a long way off. Increasing the installation size sufficient for practical use is still a very difficult task. There are many problems that need to be solved.
In particular, take into account the extreme conditions on the Moon: sudden temperature changes (from about minus 173 degrees at night to plus 127 degrees during the day), the absence of an atmosphere, deadly solar radiation and weak gravity. The lunar soil itself adds additional complexity — it is heterogeneous, and its composition can vary greatly in different regions of the Moon.
Another catch is that there is almost no carbon dioxide on the moon. And it is necessary for the reactor to start working. It can only be obtained from the air that astronauts exhale. Because of this, the installation will be able to perform its functions well only inside a closed system where everything is interconnected: waste from some processes become resources for others.
However, the fact that the device uses available solar energy and operates on real lunar soil without having to bring sophisticated equipment from Earth opens a new chapter in lunar science.
More information about the technology can be found in the article, [url=https://www.cell.com/joule/fulltext/S2542-Igor Baydov